Exploiting the potential of HoloMonitor® digital holographic microscopy by combining it with 3D matrix cell culture assays
Monica Hellesvik1,2, Hanne Øye1 and Henriette Aksnes 1
1Department of Biomedicine, University of Bergen, Bergen Norway.
2Department of Biological Sciences, University of Bergen, Bergen, Norway.
ABSTRACT
Culturing cells in a 3D scaffold provide the cells with an environment closer to actual physiological conditions than the more common 2D cell cultures. This is particularly important when studying cell movements as the higher complexity of the microenvironment affect the biomechanics. However, using gels to create the 3D environment usually causes problems for live cell microscopy since cells are found at different focal planes, whereas in 2D, all cells are in the same focal plane. Additionally, the gels themselves may disturb the imaging. Here, a different type of microscope, HoloMonitor M4, based on digital holography, has demonstrated high compatibility with 3D Matrigel cell cultures. We were able to quantify wound closure and cell movement in Matrigel by single cell tracking. We also found possibilities for further applications, such as analysis of drug response, tumor growth, metastasis, and invasion, in our 3D preparations.

By combining two powerful techniques, digital holographic microscopy using HoloMonitor M4 + 3D matrix embedded cells, we added a third dimension to our cancer cell research. This methodical advance enabled us trap & track cells in the matrix and thereby reveal morphological differences between migratory and invasive mode of cancer cells at the “leading edge”.
Monica Hellesvik,
University of Bergen
Background
It has been recognized that the cell microenvironment is of the highest importance in cancer research. Since 3D cell cultures mimic the physiological conditions more accurately than the more common 2D cell cultures, it is essential to develop methods to study 3D cultures. Matrigel mimics the basement membrane with natural proteins of the extracellular matrix. Proteolysis, integrin expression, and the formation of invading structures are some of the cellular behaviors observed during cell migration through the Matrigel matrix. The present study aimed to evaluate the use of HoloMonitor for studies of cell movements and morphology of cells grown in Matrigel 3D environment.
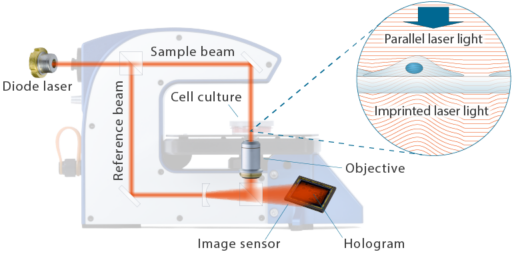
Methods
3D Cell Culture
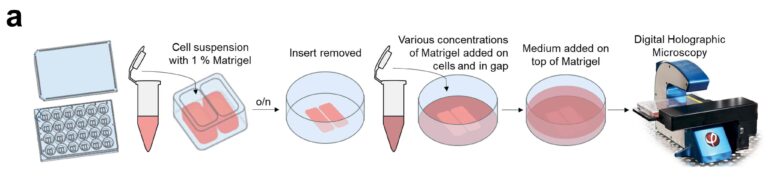
All cell culturing and all preparation procedures are outlined in Hellesvik et al (2020). Three different preparations with or without Matrigel were set up: no Matrigel (uncoated), cells in 1% Matrigel, and cells in 1% Matrigel which was then covered with 50% Matrigel. The overall workflow of wound healing assays is shown in fig. 2.
Results and discussion
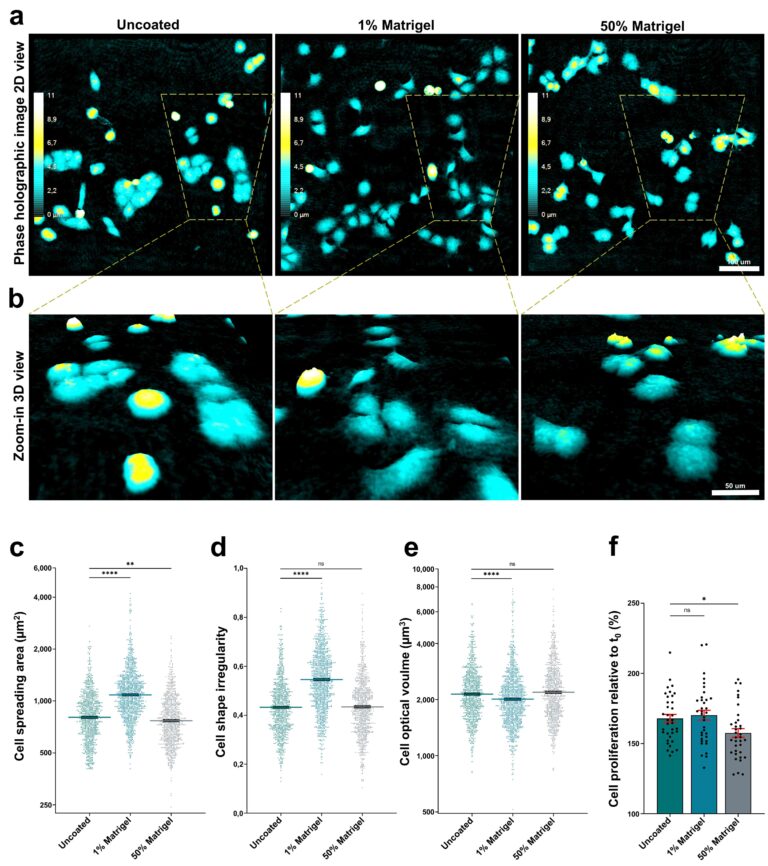
Various preparations with or without Matrigel were explored for its compatibility with the HoloMonitor system. We found that imaging quality was not notably affected by Matrigel (fig. 3a and b). Cell spreading and irregularity increased in the preparation with 1% Matrigel (fig. 3, c-e). Next, cell proliferation was analyzed using the HoloMonitor. As seen in fig. 3f, there is a slight increase in cell number for 1% Matrigel and a decrease for the 50% Matrigel samples compared to samples with no Matrigel.
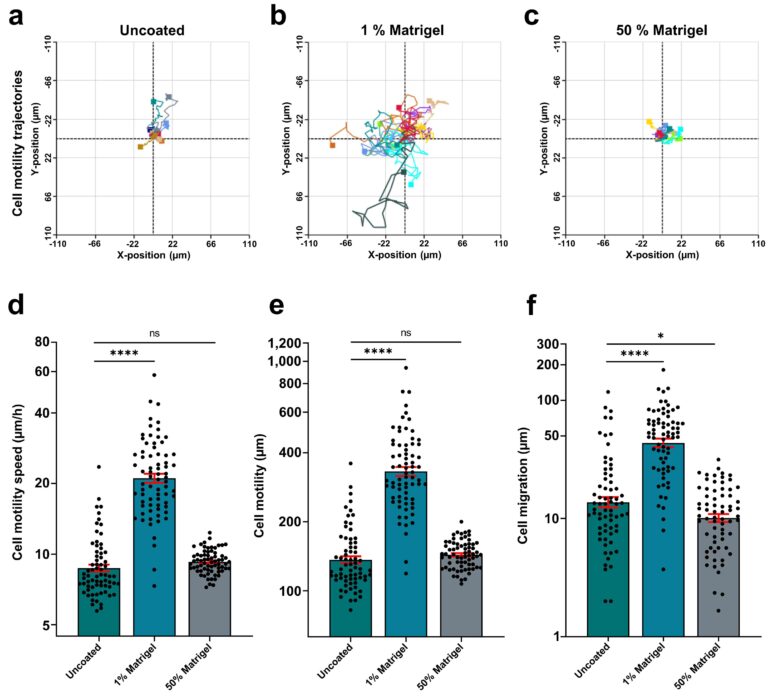
The rose plots showing individual cell tracking in fig. 4a-c demonstrate that in 1% Matrigel, cell movements increased substantially. The same applied for all cell movement parameters, like motility speed, accumulated cell motility, and cell migration in the same preparation (fig. 4 d-f).
An invasion assay was set up using ibidi wound healing inserts (fig. 2). The gap between two areas with densely packed cells was filled with 50% Matrigel. The results were compared to a standard monolayer ibidi wound healing assay. The Matrigel did not reduce the image quality (fig. 5e). In the invasion assay, the Matrigel covered gap (fig. 5, invasion) was clearly closing more slowly than the gap in the standard monolayer ibidi wound healing assay (fig. 5, migration). In addition, the preparation of suspension cells embedded in Matrigel was studied and the growth of smaller cell clusters was quantified together with cells migrating in and out of the cluster (not shown 2).
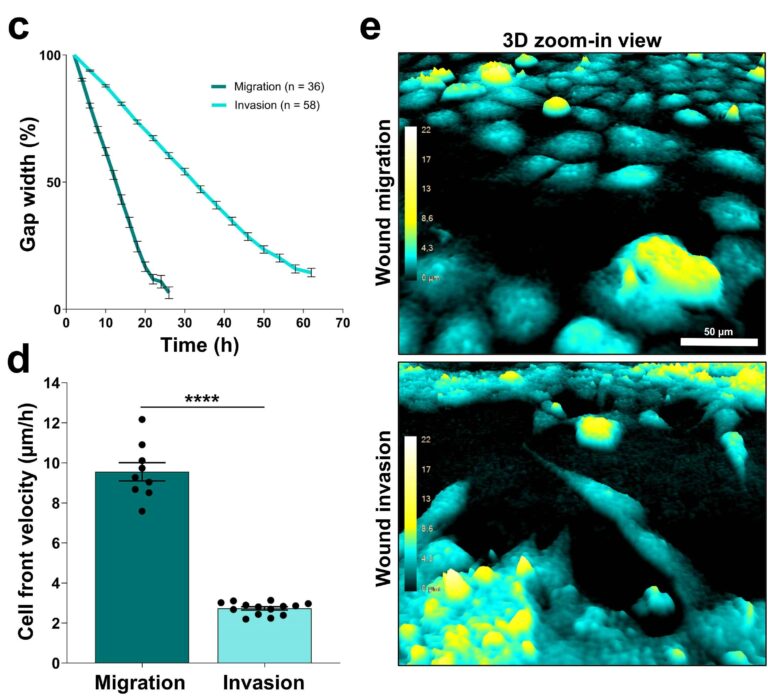
Conclusions
The HoloMonitor M4 was well compatible with the Matrigel preparations used here. There was no difference in image quality or analysis parameters even for the thickest (50-70%) Matrigel preparations when analyzing wound healing or cell invasion. Matrix-embedded suspension cells could also be investigated using the HoloMonitor by quantifying the growth of cell clumps and cells moving out of cell clumps. This study clarifies for the first time that HoloMonitor can be used for monolayer cell cultures embedded in a 3D matrix to study invasion.
References
Mölder et al. (2008), Non-invasive, label-free cell counting and quantitative analysis of adherent cells using digital holography, J. Microscopy, 232:240-247, http://dx.doi.org/10.1111/j.1365-2818.2008.02095.x
Hellesvik et al. (2020) Exploiting the potential of commercial digital holographic microscopy by combining it with 3D matrix cell culture assays, Scientific Reports, 10:14680, https://doi.org/10.1038/s41598-020-71538-1.
