Joining forces with Huntsman Cancer Institute
Quantifying epithelial-mesenchymal transition
PHI is working with scientists at the Huntsman Cancer Institute (HCI) in the battle against cancer. Supported by PHI, Dr. Robert Judson-Torres and his team at HCI aim to develop methods to quantify the phenomena known as epithelial-mesenchymal transition.
HCI is a National Cancer Institute-designated Comprehensive Cancer Center, supported by the National Institutes of Health.
Epithelial-mesenchymal transition (EMT) regulates the deadliest hallmark of cancer — invasion and metastasis. 80% of all cancer forms, including breast, prostate and skin cancer, arise from epithelial cells. Epithelial cells are located throughout the body, lining organs and blood vessels, but also our bodies; the outer layer of our skin are epithelial cells.

Commencement at University of Utah
Like normal epithelial cells, cancerous epithelial cells are rigid and immobile, incapable of creating metastasis. To become a lethal colonizer, a cancerous epithelial cell must first transition into a highly plastic and motile mesenchymal cell, hence the term epithelial-mesenchymal transition.
EMT is a physiological transformation caused by a spectrum of different biochemical processes, making the conventional approach of biochemical characterization indirect and adventurous. Contrary to conventional biochemical methods, HoloMonitor’s unique ability to non-invasively quantify physiological traits, such as single-cell plasticity and motility, allows HoloMonitor to directly and more precisely characterize EMT. Moreover, as no reagents contaminate the cells, the same cells may be reused to determine additional cellular characteristics, which is especially useful when working with scarce primary cells extracted from cancer patients.

Insight into metastatic potential provided by real-time quantification of EMT and phenotypic plasticity is conveniently achieved without labels and with single cell resolution.
Dr. Robert Judson-Torres
Robert's research interests focus on the networks of genes and environmental factors that stabilize cell states in adult mammalian organisms, and, conversely, the coordinated sets of destabilizing factors which can lead to tumorigenesis. He is also actively involved with exploring new models of scientific training, communication and publication, including experimenting with forums for post-publication peer review, reproducibility initiatives, and strategies for training the scientific workforce.
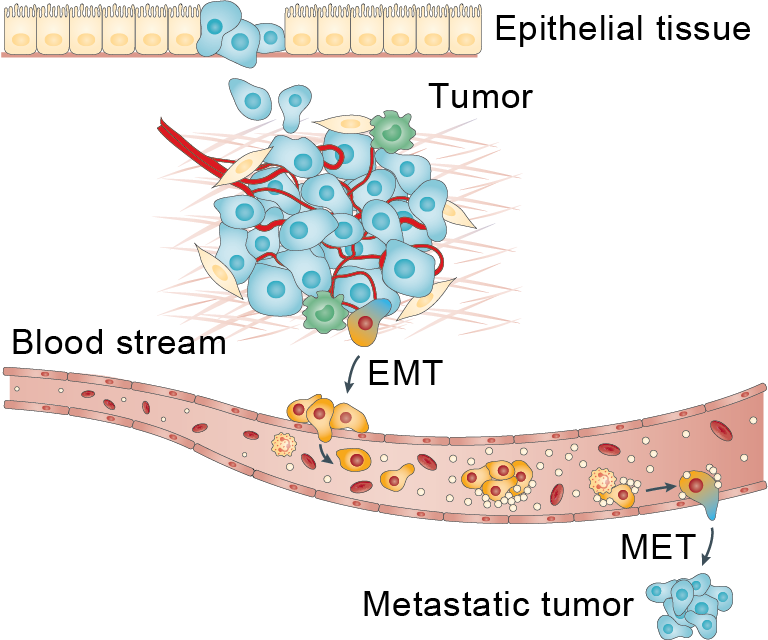
Drawing of EMT and its reverse, mesenchymal-epithelial transition (MET)
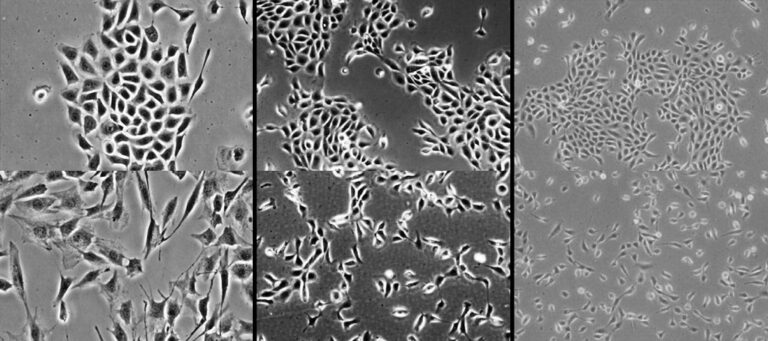
Cell images, before (top row) and after EMT (bottom row)
Read more about EMT here: The basics of epithelial-mesenchymal transition, Journal of Clinical Investigation (2009).
Peer Reviewed Articles and Book Chapters

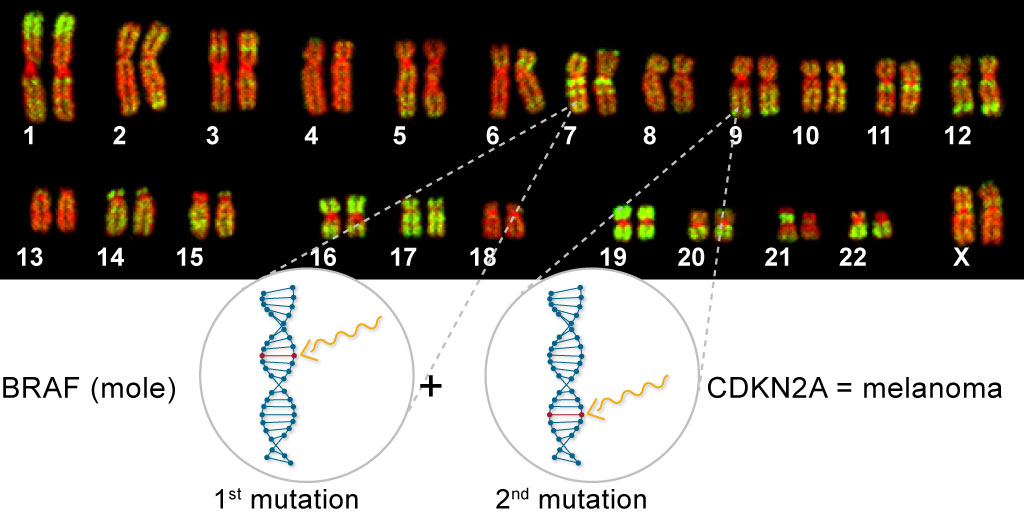
Bi-allelic Loss of CDKN2A Initiates Melanoma Invasion via BRN2 Activation
Authors: Hanlin Zeng et al.
Journal: Cancer Cell (2018)
Research Areas: Cancer research
Cell Lines: Melanocytes
Keywords: HoloMonitor M4, Cell division, Cell death, Senescence, Cell motility, CDKN2A, Melanoma, Invasion, Melanocytes, CRISPR engineering, E2F1 BRN2

Quantification of mammalian tumor cell state plasticity with digital holographic cytometry
Authors: Miroslav Hejna et al.
Journal: SPIE Conference Proceedings (2018)
Research Areas: Cancer research
Cell Lines: Human melanoma cell
Keywords: HoloMonitor M4, Digital holography, Image segmentation, Melanoma, Holography, Tumors, Cancer
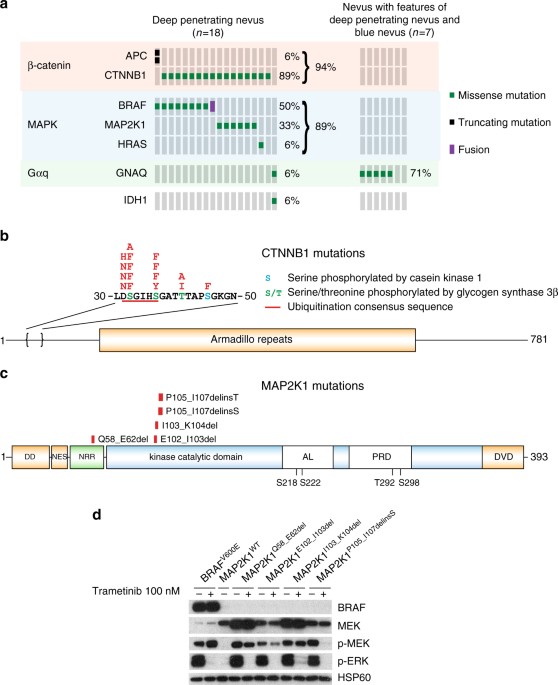
Combined activation of MAP kinase pathway and β-catenin signaling cause deep penetrating nevi
Authors: Iwei Yeh et al.
Journal: Nature Communications (2017)
Research Areas: Cancer research
Cell Lines: Melana-a
Keywords: HoloMonitor M4, Cell morphology, Deep penetrating nevus, Melanoma
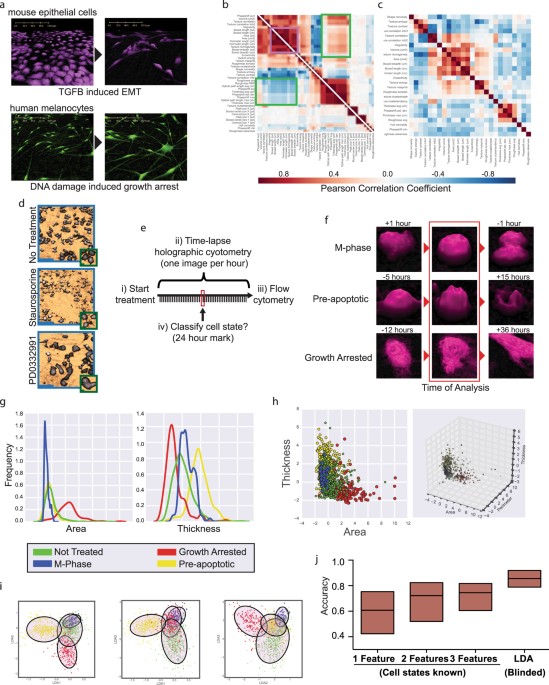
High accuracy label-free classifcation of single-cell kinetic states from holographic cytometry of human melanoma cells
Authors: Miroslav Hejna et al.
Journal: Scientific Reports (2017)
Research Areas: Cancer research
Cell Lines: A375
Keywords: HoloMonitor M4, Cell morphology, Melanoma
Interviews and News
Cell Tracking for Early Skin Cancer Detection
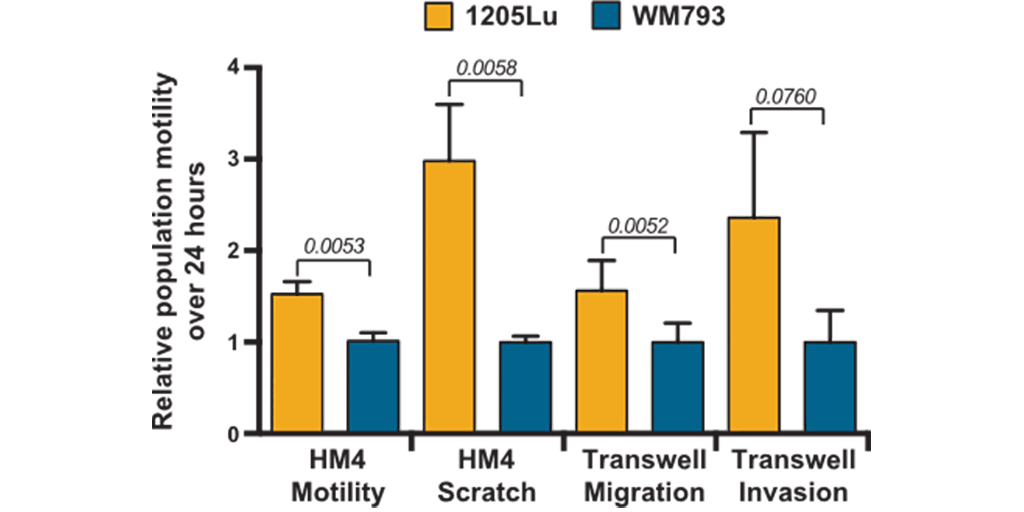
New Publication shows Advantages of using HoloMonitor® for Cell Movement Assessment