created by HoloMonitor®
Label-free Live Cell Imaging
Live cell imaging employing phase microscopy techniques facilitate non-invasive label-free analysis of living cells in real-time. Powered by sophisticated time-lapse imaging software, these novel 3D imaging systems allow long-term live cell visualization and analysis of in vitro cell culture populations on a single-cell level — without compromising cell behavior.
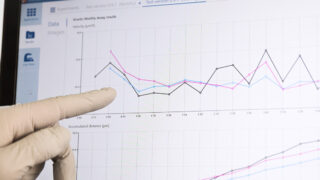
When watching a time-lapse movie of a thriving cell population, it becomes evident that cellular interactions and individual cell behavior are far more elaborate and heterogeneous than we currently appreciate.
Automated live-cell microscopy promise to help us understand the contribution of single-cell behavior to the overall response of in vitro cell populations. This new generation of imaging microscopes emphasizes physiological relevance and cellular dynamics by allowing live cells to be non-invasively imaged and quantified directly in an incubator environment over long time-periods.
A time-lapse movie of a thriving cell population. The label-free video was recorded over 48 hours from within a cell incubator, using the HoloMonitor live-cell imaging microscope.
Sebesta et al., HoloMonitor M4: holographic imaging cytometer for real-time kinetic label-free live-cell analysis of adherent cells, Proceedings, Quantitative Phase Imaging II (2016)
The HoloMonitor live cell imaging system allow individual cells to be non-invasively visualized and quantified over time on a population level.
The Live Cell Imaging Predicament
Living mammalian cells are as translucent as ice cubes in water. To be visible in a standard or fluorescent light microscope, cells must, therefore, be stained or genetically modified to absorb, emit or scatter light. Unfortunately, the invasive preparations necessary to make cells visible are likely to affect cellular behavior, compromising the in vivo relevance of in vitro live cell observations.

Gentle Cell Imaging Techniques
Unstained cells do, however, slow down and distort the light passing through them, just like beach waves are distorted by shallower water (below). By using a phase contrast microscope these phase-shift distortions created by living cells can be observed, making unstained cells clearly visible.
Just like water waves, light waves of a specific wavelength have two principal characteristics: amplitude and phase. Amplitude corresponds to light intensity and is the height of the wave, measured from crest to trough. Phase describes whether a wave is currently at its crest, in its trough, or somewhere in between.
When light passes through a cell submerged in cell media, the light amplitude is unaffected. But the more optically dense cell slows down the light slightly relative to the surrounding ambient light.
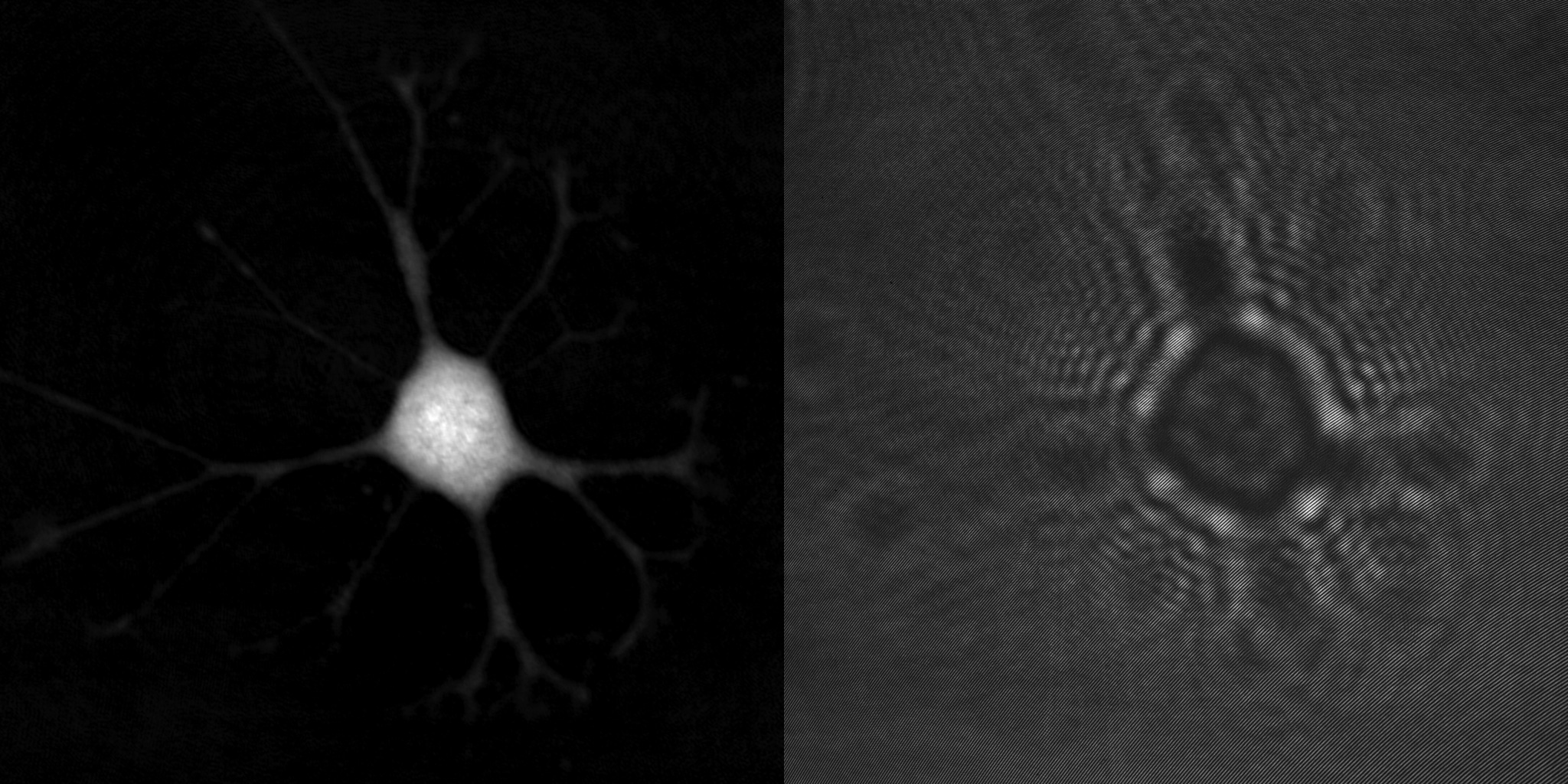
This speed reduction creates the relative phase-shift that makes unstained cells visible in a phase contrast microscope. However, conventional phase imaging techniques are non-quantitative. They only visualize the phase-shift created by a cell by making edges and its perimeter more prominent.


Phase-shift distorted beach waves, created as a result of the lower wave speed in shallower water.
Quantitative Phase Imaging
Using a digital image sensor, low power diode illumination and sophisticated computer algorithms, the HoloMonitor live cell imaging system has the ability to both quantify and visualize phase-shifts.
The HoloMonitor system employs a live cell imaging technique called quantitative phase imaging (QPI) or quantitative phase contrast microscopy, to distinguish it from its soon 100-year-old non-quantitative predecessor — the phase contrast microscope. QPI’s capacity to quantify phase-shifts allows QPI to image and visualize cells in 3D by recreating the imprint a cell creates on the illuminating laser light when it passes through the cell.

Monica Hellesvik, Hanne Øye & Henriette Aksnes, Exploiting the potential of commercial digital holographic microscopy by combining it with 3D matrix cell culture assays, Scientific Reports (2020)
See also the blog piece A LOCKDOWN EXPERIENCE — Research from home, commenting the above work.
Gentle Time-Lapse Imaging
As the cell does not absorb any light energy, the cells are completely unaffected when observed using HoloMonitor — no energy exchange, no change. This allows HoloMonitor to gently acquire time-lapse image sequences over extended periods of time without compromising cellular behavior.
Within the incubator, HoloMonitor provides both quantitative and beautiful time-lapse images of living cells. This ability transforms live-cell microscopy into a quantitative tool for detailed population analysis of live cells on a single-cell level.
Sophisticated Cell Imaging Software
The HoloMonitor cell imaging software offers a range of label-free imaging applications to allow the dynamics of cell populations to be non-invasively studied on a single-cell level over time:
- cell morphology,
- cell proliferation,
- cell motility and migration,
- cell invasion,
- cell differentiation,
- cell cycle phase and other cellular events.
Live-Cell Microscopy — First Point of Investigation
As the time-lapse analysis leaves the cells completely unaffected, samples may be reanalyzed using complementary downstream instrumentation, such as confocal fluorescence microscopy and other more invasive methods.

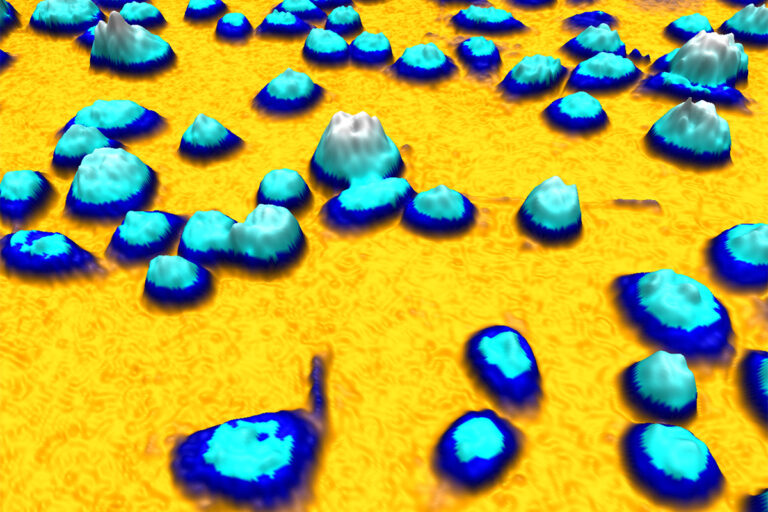
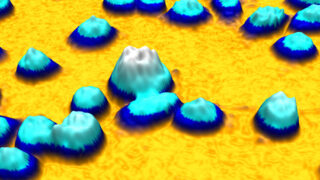
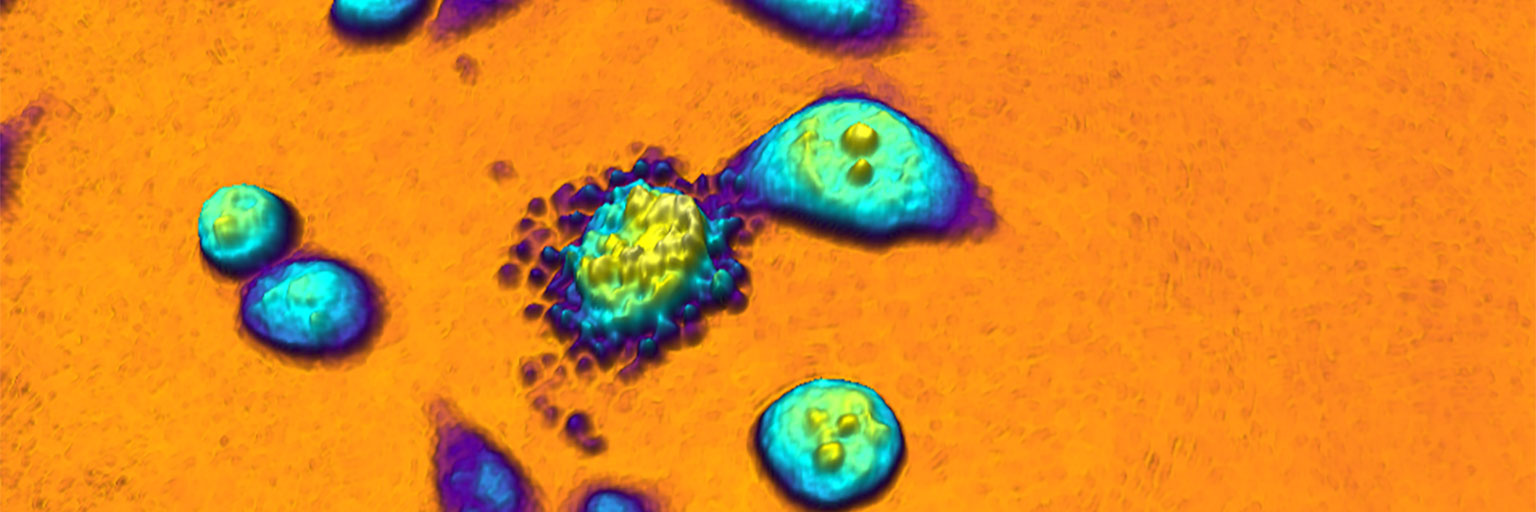
An example of a quantitative phase image of living cells in 3D created by HoloMonitor while operating inside a cell incubator. The height of the cell and its color tone correspond to the optical thickness of the cell.
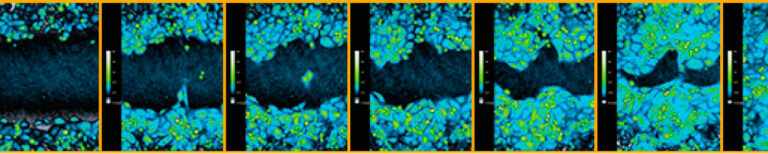
Time-lapse image sequence created when using the HoloMonitor Wound Healing Assay.