
Q&A Investor Relations
Below you will find frequently asked investor questions and our CEO’s answers
Latest edition September 12, 2024
Investor FAQ update to include your questions
We’re updating our Investor FAQ to include your questions about PHI AB. Please send us your queries and help shape our FAQ update.
TO 4 warrants
How do I exercise my TO4 warrants?
Important dates
- 12 September 2024: Exercise period commences
- 1 October 2024: Last day of trading in TO 4
- 3 October 2024: Exercise period ends
- 4 October 2024: Planned date for publication of outcome of the exercise
- 18 October 2024: Planned date for change from interim shares to shares
There are 1,346,162 issued warrants of series TO 4. Holders of warrants of series TO 4 have the right to subscribe for one (1) new share in Phase Holographic Imaging PHI AB (“PHI”) at a price of SEK 4.63 per share for each warrant. Subscription with the support of warrants of series TO 4 can take place during the period from and including 12 September 2024 to and including 3 October 2024. Subscription must take place by simultaneous cash payment no later than 17:00 CET on 3 October 2024.
Where do I find more information regarding the TO 4 warrants?
TO 4 in short
- Exercise price: Each TO 4 entitles the warrant holder to subscribe for one (1) new share in PHI at a price of SEK 4.63 per share.
- Issue volume: There are 1,346,162 issued TO 4. At full utilization of TO 4, PHI receives approx. SEK 6.2 million before issue costs.
- Number of outstanding shares prior to warrant exercise: 26,192,925 shares.
- Short name and ISIN: TO 4 are traded at Spotlight Stock Market under short name “PHI TO 4” and with ISIN SE0017766462.
Complete terms and conditions for warrants of series TO 4 are available on the Company’s website (www.phiab.com). An information sheet containing summary information about the warrant exercise is also available on PHI’s, Sedermera Corporate Finance AB’s (www.sedermera.se) and Nordic Issuing AB’s (www.nordic-issuing.se) respective websites.
What is the time plan for the TO 4 warrants, and how are you going to use the proceeds?
Upon full utilization of TO 4, the number of shares in PHI will increase by 1,346,162 shares to a total of 27,539,087 shares and the share capital will increase by SEK 269,232.40 to SEK 5,507,817.40. The dilution at full utilization amounts to approximately 4.9 percent of the capital and votes.
The proceeds from the TO 4 warrants are intended as investments in our regenerative medicine initiatives and in enhancing our ongoing product development. The proceeds continue the momentum from previous funding rounds, which started with the rights issue in 2022 and then continued with the TO 3 warrants in 2023. Our strategic partnership with Altium to scale our sales processes and global distribution networks allows us to focus in-house on our innovation capabilities and product development and drive our mission in regenerative medicine.
US OTQCB dual-listing
Since when has PHI been listed on a US stock market?
Since August 15th, 2024, PHI has been dual-listed on the US-based OTCQB Venture Market.
Additional reading

PHI enters the US capital market – shares now also traded on OTCQB

BioStock: PHI’s CEO comments on the approved US listing
OTC Market Center in New York welcomes PHI
Product Development

Do you have any plans to develop HoloMonitor further after the fluorescence module?
Yes!
What is the advantage of combining holographic imaging with fluorescence imaging?
The combination makes it easier and less expensive to gain fluorescence insights. Coupled with that, it minimizes the harmful effect of fluorescence labeling on the cells. Especially, reduced cell impact is very important to produce reliable research results, which is important to our users.
Which of your customers request the combination?
Almost all of our users ask about fluorescence. Cell biologists are very interested in the genetic activity of cells. To some extent, it is possible to see genetic activity with holography, as the activity results in changing the shape or behavior of the cells. However, fluorescence microscopy is needed to see more subtle biochemical changes.
Will the customers who have already purchased the HoloMonitor be able to add the fluorescence module afterward?
Yes, the HoloMonitor M4 system has been prepared for a fluorescence add-on for a long time.
Regenerative Medicine
“We envision that PHI and our key technology are integrated into established quality standards within regenerative medicine.” – What would that entail for your sales and finance? And how would that look in praxis?
A quality standard means that all companies acting within regenerative medicine must use the established standard for quality control and approvals. Thus, the first companies creating that standard have a competitive edge in the market.
For PHI technology to integrate into the quality control process in regenerative medicine, do you need to make any changes to the current M4 hardware and software?
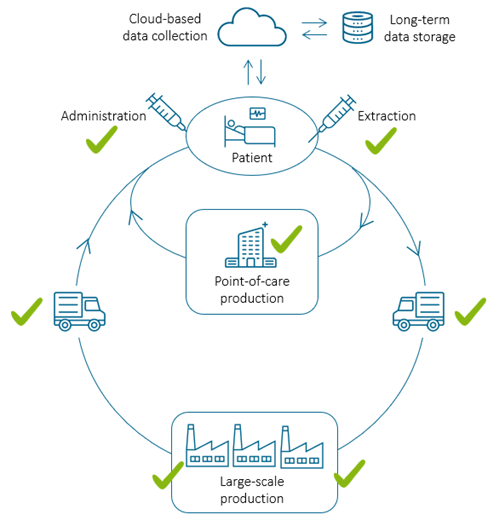
Yes, to integrate our technology into manufacturing processes, the system will most likely be tailor-made. Generally, at the patient bedside and for cell transport, we envision a standard system, most likely a development of the present HoloMonitor product line.
GlycoImaging
Read more about GlycoImaging in the article Fighting cancer at an early stage.
What is the status of the MIPs patent?
We secured the synthetic antibodies patent in the US, Japan, and the EU.
What is the plan for the MIPs patent?
We have established a dedicated subsidiary, PHI MIPS AB, to house the synthetic antibody patent family and facilitate its business potential. This subsidiary provides transparency regarding the synthetic antibody patents, setting it apart from PHI AB’s core business. Overall, the idea is to license out the patent right or sell the patent.
Communication
How can I stay informed about the Company?
- View PHI’s profile and see the share price on Spotlight Stock Market.
- Subscribe to the latest PHI news from BioStock, Kalqyl and Cision to stay informed.
- Visit our phiab.com investor pages, which we regularly update with information.
- Follow PHI on social media, where we keep in touch with our global HoloMonitor community.
