About PHI

With our HoloMonitor® products, we transform how researchers study living cells and perform live cell analysis in life science and medical research.
We empower any lab to work cell-friendly by providing innovative tools with proven QPI technology for non-invasive imaging and analysis of living cells.
We envision QPI technology as a standard method for cell quality control, making our future cell therapies safe, affordable, and accessible for patients.
Our QPI technology has the potential to become a future standard method for cell quality control in regenerative medicine.
PHI in brief
Phase Holographic Imaging PHI AB is a medical technology company.
We develop and market a new generation of non-invasive, time-lapse imaging instruments for quantitative and long-term analysis of living cells.
The foundation of our current commercial HoloMonitor® products is Quantitative Phase Imaging (QPI) technology, which brings an innovative approach to real-time cell quality evaluation.
It offers a detailed analysis of a large number of cell health and behavior characteristics without harming or influencing living cells. This critically differs from conventional measurement methods, which often jeopardize cell integrity.
Now, we actively focus on business development to expand from our current pre-clinical research market to the sizable healthcare industry and the emerging regenerative medicine field. We at PHI envision transforming live cell analysis and setting a new benchmark with QPI as a standard method for cell quality control, enabling regenerative treatments to advance and making future cell therapies safe, reliable, and economically and universally accessible for patients worldwide.
PHI’s headquarters are in Lund, Sweden, with offices in Boston, MA and Winston-Salem, NC.
Find your answers:
July 2024, Finwire Hemma Hos — Interview with PHI AB in Lund
Watch more video presentations about PHI
March 2023, BioStock Studios — Welcome to PHI, our company, its people and our future in regenerative medicine.
June 2022, World Stem Cell Summit conference presentation — Advanced Quality Control for Regenerative Medicine
March 2022, BioStock Investor Meeting — Empowering medical research
June 2020, Spotlight interview — Från idé till börsnotering (Swedish)
May 2019, Biostock interview — Peter Egelberg kommenterar teknikutvärderingsavtal (Swedish)
June 2018, PHI analyserar celler på cellernas villkor (Swedish)
March 2017, Småbolagsafton hos Erik Penser (Swedish)
March 2016, Sedermeradagen
(Swedish)
Background, or Our Why?
We all rely on good preclinical research
Before a new drug is clinically tested on patients, extensive preclinical research tests are carried out on cultured cells and laboratory animals. The purpose of these initial trials is to determine if the new drug has the potential to become a safe and effective drug for healthcare. Drug development is expensive — very expensive. The high costs are due to the many failures. Unfortunately, 9 out of 10 clinically tested drugs are discontinued due to lack of effect or too extensive side effects. The reason for so many failures is that the preclinical results are too flawed, so clinical trials start on false grounds.
Scientists need better data
Conventional test methods require living cell cultures to be genetically manipulated or stained with toxic substances before analysis. This limits and hinders experiments. As a result, the influence on those living cells also limits and distorts the preclinical results. They determine whether costly clinical trials should be started — a standard that requires an innovative update.
Our solution
PHI’s HoloMonitor® technology solves this dilemma by replacing a nearly 100-year-old microscope technique with a modern computer-based technique — QPI technology with holographic microscopy. It neither influences the cells in any way nor causes uncharacteristic cell behavior. It means that the measurement method is not affecting the cells. This is very relevant in drug development and basic medical research for accurate and cost-efficient research and satisfying patient outcomes.
We transform how scientists study living cells.
A common question:
What is time-lapse imaging?
Time-lapse imaging means that living cells are continuously photographed over time. Then, the image sequence is fast-forwarded to study the slow growth, movement or behavior of the cells.
Our Products
More than 200 HoloMonitor® live cell analysis systems have so far been delivered worldwide to our customers across academia and industry. Some of our more well-known customers include Bayer AG, the National Institutes of Health, Harvard University, Stanford University and Novo Nordisk A/S.
We at PHI have been granted over 20 patents in six different families and have a pending portfolio of additional applications. Furthermore, the company’s customers have published over 300 scientific articles based on data from HoloMonitor.
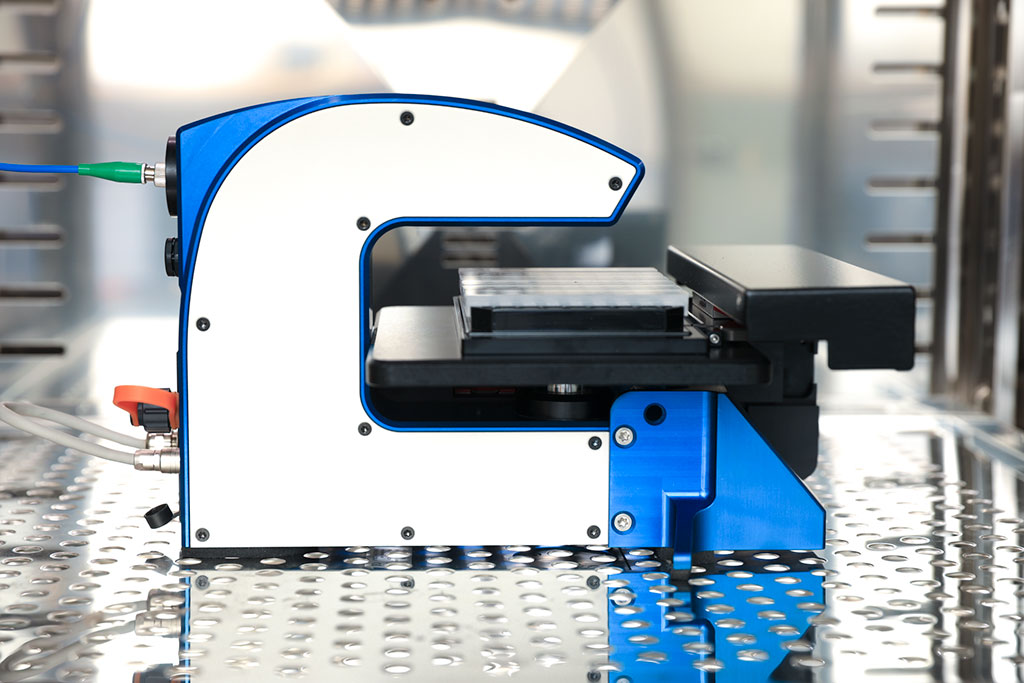
HoloMonitor® M4
Unlike conventional cell analysis instruments, the HoloMonitor® live cell microscope is designed to be placed inside the cell incubator where cells are grown. This allows researchers to monitor their cells in an optimal culture environment continuously. Furthermore, our pioneering technology makes it possible for scientists to study the behavior of living cells without disturbing them. This has previously been difficult to do. Conventional techniques require the cells to be genetically manipulated or stained with toxic dyes, altering the cellular behavior the researchers wish to study.
Learn more about our Quantitative Phase Imaging (QPI) Technology

Traditional microscopy techniques image cells by recording the intensity of light. However, human cells are transparent, which means they neither absorb nor scatter light. This makes them as difficult to see under a traditional microscope as a cube of party ice in a water glass — the clear ice cubes we never manage to make at home.

Shallow water around a sandbar slows down ocean waves in the same way cells delay light waves as they pass through. PHI’s microscope technology measures this delay and makes it possible to image and measure unaffected cells without the genetic manipulation or toxic staining used by conventional microscopy and cell analysis methods.

When two light beams meet, light creates a wave pattern like water waves. In PHI’s HoloMonitor® system, a laser beam is split into two. One beam passes through the cells while the other passes by the sample as a reference. When the beams meet, a wave pattern (hologram) is formed, which contains information about how much the laser light was delayed when it passed through the cells.
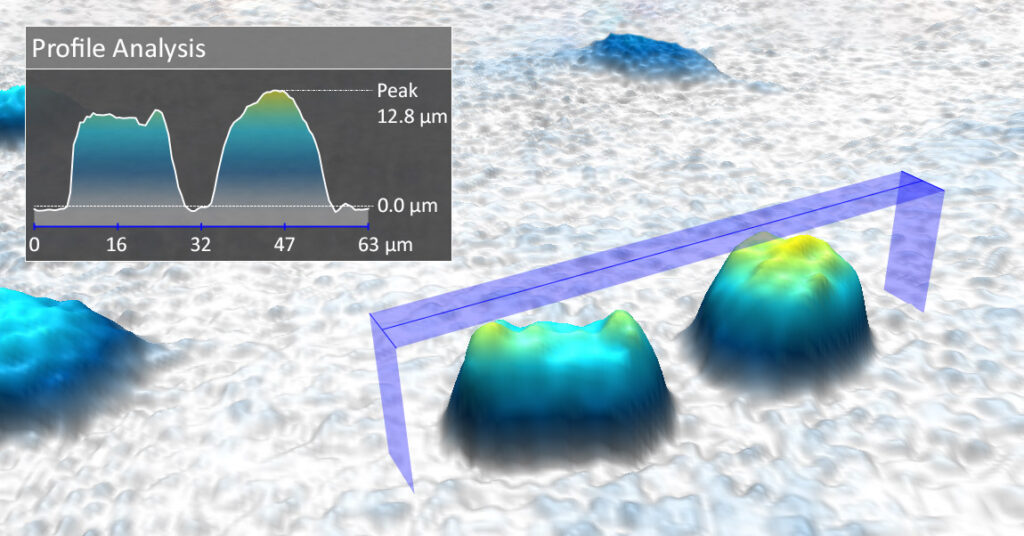
The hologram is photographed with a digital image sensor. From the information in the hologram, 3-dimensional images of the cells are computer-calculated, where the height and color of the cells reflect the delay. The holographic microscope technology makes it possible to measure cell volume and other important properties that cannot be measured with conventional microscope technology.
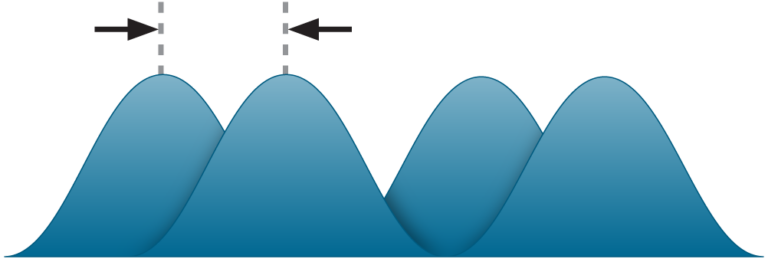
The delay causes the light waves to shift relative to each other. PHI’s holographic imaging technology images living cell cultures through this phase shift. Hence, the company’s name is Phase Holographic Imaging.
App Suite software and Live Cell Assays
App Suite is the tailor-made HoloMonitor® cell imaging software based on biological applications. The software has an intuitive user interface that guides the user through a simple step-by-step workflow, from setting up the experiment to presenting the results. The software also includes multiple Live Cell Assays, allowing users to study multiple cellular events easily. Here, the user benefits from a simple and time-saving workflow, as they can extract several results from the same experiment.

Fluorescence module
Fluorescent labeling of cells is today the best method for studying the genetic activity of individual cells. However, the method is toxic to the cells and can changes the behavior that is intended to observe. To make new research tools available to the medical research community, PHI has developed an additional fluorescence module compatible to the HoloMonitor® M4 system.
Accessories
We offer our customers further accessories to streamline and simplify their workflow. Cells grown in a nutrient solution at the bottom of different types of plastic containers. We developed a special HoloLids™ to easily avoid the condensation that forms with conventional lids. In addition, we have Vessel holders to fit different vessels to the HoloMonitor® system and HoloDry™ to keep the system’s optics and interior dry when operating inside a cell incubator.
Our mission is to empower any lab to work cell-friendly by providing the next generation of innovative tools for non-invasive imaging and analysis of cells.
HoloMonitor is very affordable. It is one of my best investments so far. The spectrum of applications is endless. I am fully convinced that this is the beginning of a new era for research.
Alain Geleon,
National Institute of Applied Sciences, Lyon

Our operations and markets
HoloMonitor® technology is proven — worldwide
PHI has been active within the preclinical and biomedical cell research market. We have established a global presence with HoloMonitor® systems and their scientific validation in both academia and industry. HoloMonitor® systems are in operation in Europe, North America, Asia and Australia, and their users have published data in more than 300 peer-reviewed scientific articles.
HoloMonitor® primarily addresses cancer research. Customers also have applications in other research areas, including inflammatory and autoimmune diseases, stem cell biology, gene therapy, diabetes, toxicological studies and cell-based drug development.
Learn more about our Business model & Strategy
We advance cell-based preclinical research …
Currently, our customers are in cell-based preclinical research — modern pharmaceutical research and medical research across academia and industry — as many questions in this field require answers from living cell culture analysis experiments.
Our HoloMonitor® technology is transforming preclinical research by providing a foundation for better cell models. Those are critical before clinical drug testing and for reducing the need for animal test models. This innovative QPI technology ensures that cells remain unaffected during analysis. By enabling scientists to obtain better data without compromising cell health, HoloMonitor® sets a new standard in drug development and basic medical research. And therefore aiming for more successful patient outcomes and reduced research expenses.
A common question:
What is preclinical research?
Preclinical research is medical research that is not performed on humans. Both animals and laboratory-grown cells are used to develop new treatment methods before they are tested on humans. PHI addresses cell-based preclinical research organizations, including universities, biotechnology and pharmaceutical companies worldwide.
… and now we expand to clinical applications
PHI is strategically prepared to extend its reach into the large clinical market and emerging fields of regenerative medicine and cell therapies. This presents significant growth opportunities. By striving to achieve Good Manufacturing Practice (GMP) standards and create a company quality management system (QMS), we aim to penetrate these markets in the future. And our non-invasive cell analysis solutions can offer critical cell quality control assessment.
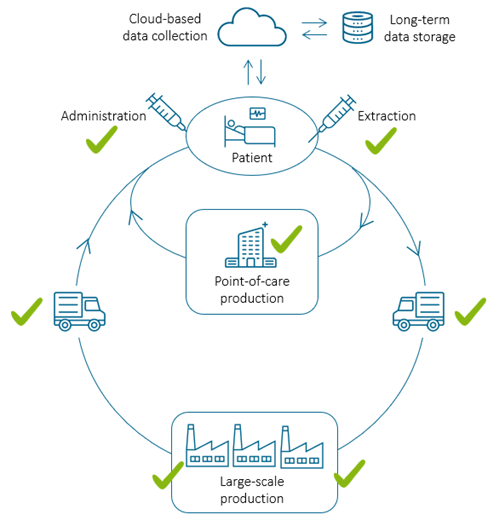
Many consider the area where laboratory-grown cells or organs themselves constitute the treatment as the third pillar of medical treatment or the future of medicine. Thus, regenerative medicine is challenging the traditional healthcare pillars — drugs and surgery. However, standardized quality control and patient safety must be improved to industrialize regenerative medicine. When regenerative therapies are accessible to everyone, QPI for standardized cell quality control would be needed in all regional hospitals and small- and large-scale manufacturing plants.
Our HoloMonitor system provides detailed cell information to assess the
quality of individual cell samples. Imagine a future where researchers
can take a patient’s cell sample at the bedside in the hospital and
instantly determine its suitability for a clinical trial or cell therapy
manufacturing.
PHI in Regenerative Medicine
In 2022, we joined the RegenMed Development Organization (ReMDO) located in Winston-Salem, North Carolina. PHI also joined an alliance to advance cell-based biomanufacturing with other companies in ReMDO and the pioneering Wake Forest Institute for Regenerative Medicine (WFIRM). Together, we aim to automate the manufacturing process of cell-based therapies. We utilize recent technological developments in cell culturing, analytical instrumentation, and artificial intelligence. We also opened a new development office in the Innovation Quarter in Winston-Salem. This way, we are present at the epicenter of this rapidly growing field.
Since 2023 PHI has been an active member of the leading international Alliance for Regenerative Medicine (ARM) and the national ATMP Sweden network. These organizations enables us to advocate for QPI technology for standard cell quality control in regenerative medicine.

A common question:
What is regenerative medicine?
Regenerative medicine is a groundbreaking field focused on developing methods to regenerate, repair, or replace damaged cells, tissues, or organs. It integrates biology, chemistry, computer science, and engineering to develop treatments for conditions previously thought untreatable.
It has already begun to transform healthcare by offering new hope to patients with conditions like cancer, Parkinson’s disease, diabetes, and deafness, displaying its vast potential to improve and save lives globally.
Our unique technology is key
Gene and cell therapies involve extracting cells from a patient or donor, modifying and multiplying the cells in a laboratory before the cells are transplanted back into the patient.
Advanced quality control is key for cell therapy to become a safe treatment. Because the cells are returned to the patient, no foreign substances may be added for quality control. However, traditional measurement methods require added reagents. Our non-destructive cell analysis technology is considered one of the few methods that can potentially become the method used to quality-assure future cell therapies.
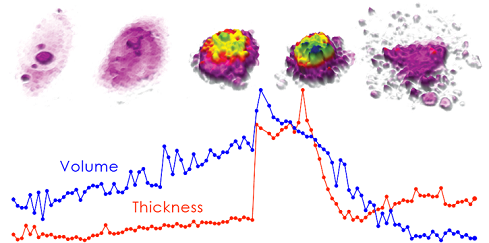
It is well known in the field that cell morphology changes when cell quality deteriorates. QPI technology generates vast amounts of data for precise cell counting, viability checks or assessing individual cell morphology that may be predictive of cell quality, ensuring the quality of therapeutic products.
The implementation of non-invasive imaging in clinical biomanufacturing would assure product consistency, save money by identifying deficient products early in the production process and assure product safety.
Joshua Hunsberger, PhD
Chief Technology Officer at ReMDO
QPI technology meets the pressing demand for non-invasive, standardized quality controls across the whole cell therapy manufacturing cycle. It addresses significant gaps and challenges in current methodologies, which is crucial for the field’s advancement as regenerative therapies rapidly evolve.
QPI has the potential to become a standard analysis method in every instance where cell-friendly quality control of live cells is needed.
We envision QPI becoming a standard method in cell quality control, making cell therapies safe, affordable and accessible for patients.
Recent news regarding regenerative medicine
Webinar series on Smart Manufacturing by the Regenerative Medicine Manufacturing Society (RMMS) — part 3 with our CSO, Dr. Kersti Alm


Shaping the future of Regenerative Medicine – PHI’s vision for QPI in Cell Therapy Manufacturing

BioStock: PHI “It’s the right moment to capitalize on regenerative medicine momentum.”
Key Figures in latest Reports

Interim Report 2 2025/26
Interim Report 1 2025/26
Future Goals
PHI is focusing on achieving important milestones in the coming years, utilizing the potential of its partnerships and collaborations. PHI has a clear focus on expanding its business to the clinical research market and positioning the company in the regenerative medicine field.
The collaboration with Altium is instrumental in PHI’s approach to gaining a stronger foothold in the regenerative medicine field, where PHI’s cell quality control technology can meet critical industry needs.
PHI is also actively fostering alliances with leading institutions such as the Wake Forest Institute for Regenerative Medicine, which enriches its initiatives and provides access to an exceptional ecosystem of expertise and innovation. This partnership bolsters PHI’s leadership in regenerative medicine, enabling the ongoing development and application of PHI’s cell quality control technology.
PHI engages in these strategic relationships to fortify the Company’s position as a thought leader in the field, accelerate progress, bolster market penetration, open new doors to new business opportunities, and enhance shareholder value.
PHI enters the US capital market – shares now also traded on OTCQB
We are excited to expand our market presence by listing on the OTCQB. This new platform offers our investors greater accessibility and visibility in the U.S. market, aligning with our mission to advance regenerative medicine. This dual listing is expected to enhance liquidity, provide greater value to our shareholders, and support the continued growth of PHI.
Patrik Eschricht, former CEO PHI
PRESS RELEASE (August 16, 2024)
Further reading to follow our journey

BioStock: PHI accelerates growth with record order

BioStock: PHI’s CEO comments on the approved US listing
OTC Market Center in New York welcomes PHI

Interview with John Moore

Why invest in PHI?
July 2024, Finwire Hemma Hos — Interview with PHI AB in Lund
- We have deep knowledge based on more than 20 years of experience. Our team of experts, scientists, and engineers creates our offering to advance cell research and enable the future of medicine.
- We have a proven technology that is transforming live cell research. Our products are established worldwide and have been used in more than 300 scientific articles.
- We have a proven track record, long-standing relationships and the trust of our customers. Nevertheless, we are continuously expanding our global network and partnering with more experts and leading institutions.
- We are well-positioned for growth as a company. Through strategic partnerships and projects, are already operating in the middle of the regenerative medicine field.
- We are continuously growing and evolving. Indeed, we prioritize yearly investments in our products, patents, and R&D. We are proud never to stand still in developing and adding to our product portfolio to meet our market’s evolving needs.
- We are on our feet to adapt to a dynamic business environment. Our quick embrace of digital tools in our sales and customer support has made us pioneers in our field in recent years.
- We have a strong vision that drives us every day. Equipped with this motivator, we have our eyes set on the future to become part of the solution for standardized quality control in the cell therapy manufacturing process.