Shaping the future of Regenerative Medicine – PHI’s vision for QPI in Cell Therapy Manufacturing
Phase Holographic Imaging (PHI) is pleased to present a comprehensive opinion piece detailing the versatility and potential the Company sees for Quantitative Phase Imaging (QPI) technology in Regenerative Medicine. PHI outlines how QPI will offer enhanced cell quality control in the cell manufacturing process, thereby addressing a central puzzle piece for making cell-based therapies safe, affordable, and accessible for all. This perspective piece aims to engage stakeholders and portray PHI’s future in the rapidly evolving and dynamic field of regenerative medicine. By sharing the Company’s vision for QPI technology, PHI aims to foster discussions, potential collaborations and anchor PHI as a thought leader in the field, opening doors to new business opportunities.

Through its notable collaborations with the Wake Forest Institute for Regenerative Medicine (WFIRM) and the Regenerative Medicine Development Organization (ReMDO), PHI has solidified its status as a key innovator for including Quantitative Phase Imaging (QPI) in advanced cell therapy manufacturing workflows. PHI leads the Smart Manufacturing project at WFIRM, aiming to create a system for cell quality control and data storage, in collaboration with the technology development alliance with ReMDO, analytics leader SAS, BioSpherix, and QIAGEN.
QPI technology, the foundation of PHI’s current commercial HoloMonitor product, heralds a new era in cell quality evaluation providing non-invasive, detailed analysis of cell characteristics without harming cell integrity, diverging from conventional methods that often jeopardize cell health.
PHI envisions its QPI technology becoming a future standard method for cell quality control in regenerative medicine. It highlights the technology’s capacity to generate vast amounts of data for precise cell counting, viability checks or assessing individual cell morphology that may be predictive of cell quality, ensuring the quality of therapeutic products.
QPI technology meets the pressing demand for non-invasive, standardized quality controls across the whole cell therapy manufacturing cycle, thus addressing significant gaps and challenges in current methodologies. As regenerative therapies rapidly evolve, addressing these challenges is crucial for the field’s advancement.
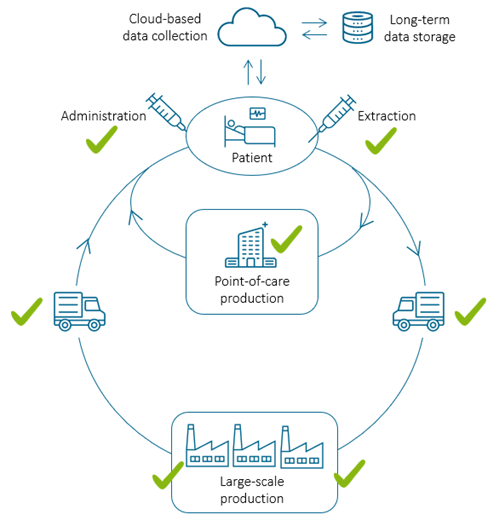
PHI’s initiative to set new standards, in collaboration with forward-thinking technology companies, represents a strategic leap towards shaping the future of regenerative medicine. Concentrating on non-invasive, real-time quality and process monitoring, PHI’s goal is to enhance the accessibility of affordable cell therapies, ensuring patient safety and treatment outcomes. Ultimately, PHI is not just envisioning a future for regenerative medicine but actively shaping it.
What is regenerative medicine?
Regenerative medicine is a groundbreaking field focused on developing methods to regenerate, repair, or replace damaged cells, tissues, or organs. It integrates biology, chemistry, computer science, and engineering to develop treatments for conditions previously thought untreatable. It has already begun to transform healthcare by offering new hope to patients with conditions like cancer, Parkinson’s disease, diabetes and deafness, showcasing its vast potential to improve and save lives around the globe.
Subscribe to PHI news on cision.com

