Automated Cell Proliferation
The automated cell proliferation assay assesses adherent cell growth by providing cell count and confluence growth curves in real-time. The walkaway cell proliferation protocol involves three simple steps:
- Sample preparation
- Experiment setup
- Automated imaging while the results are shown in real-time
Cell proliferation assays are widely applied in biomedical sciences, especially for potency and cytotoxicity testing of drugs and chemicals. The assays are commonly used to understand how drug dose levels affect cells over time, typically by creating cell growth curves to determine the inhibitory concentration at half maximum effect (IC50).
Walkaway Cell Proliferation
In Your Incubator
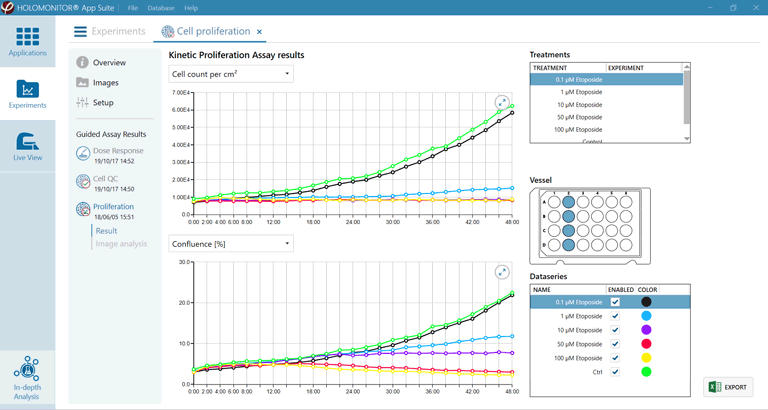
- For various microplate formats, the HoloMonitor proliferation assay conveniently records non-invasive quantitative time-lapse images of multiple cell cultures inside your incubator.
- While the time-lapse image sequences are automatically recorded, the assay presents real-time growth curves by cell count (top) and cell confluence (bottom) at user-selected intervals.
Cell Proliferation FAQ
Why measure cell proliferation using HoloMonitor?
The HoloMonitor® kinetic proliferation assay is non-invasive and needs no staining or labeling. The analyses are performed on adherent cells in standard cell culture vessels, making trypsinization redundant. The same sample is used throughout the experiment, substantially reducing the number of samples needed.
Why are conventional proliferation assays less reliable?
There are many methods to measure cell culture growth. The standard methods that aim to measure cell numbers, e.g. MTT, XTT or AlamarBlue cell proliferation assays are indirect. They are based on cell metabolism and measure different metabolic end products. It is assumed that the more end product that is measured, the more cells there are. These biochemical methods will give incorrect results when the tested substances cause increased metabolism or cell size.
OK, but what about assays based on direct cell counting?
Both indirect and direct assays based on cell counting are destructive end-point methods, where the cells are discarded after the measurement. The methods, therefore, rely on multiple parallel samples to create a growth curve. If the parallel samples are not identical at the starting point, just a slight difference in seeding numbers may lead to major differences after some time.
What proliferation measurements are provided by the assay?
The assay provides real-time growth curves by cell count and cell confluence at user-selected intervals.
Can the recorded data be reused to analyze additional cellular properties?
Certainly, as the HoloMonitor live cell imager assess cell culture growth non-invasively and does not require assay-specific sample preparation, the recorded time-lapse image sequence can be reused to determine other cellular characteristics associated with potency and cytotoxicity. Using, for example, the HoloMonitor Cell Morphology, Cell Motility and the Dose-Response Assay, cell morphology, motility and dose-response can also be quantified without requiring additional experiments, saving precious cells and lab hours.
Can the sample be reused for additional experiments using complementary instrumentation?
Yes, again as growth assessment is done non-invasively and without assay-specific sample preparation, the sample can be used in further downstream experiments using any type of instrumentation.
Does this mean that the proliferation assay can be used for cell QC as well?
Yes, as the results are displayed in real-time, the assay can be used to grow a cell culture to a specific cell density. Besides the proliferation assay, we also offer a specific Cell QC assay.
Maximize Clinical Relevance

Cell Proliferation Protocol
The walkaway cell proliferation assay protocol is straightforward — sample preparation, experiment setup followed by automated image recording while the results are presented in real-time.
- Seeding
To assess cell proliferation, seed unlabeled adherent cells in a standard cell culture vessel, e.g. a multiwell plate or TC-dish. - Stage the Sample
Place the cell culture vessel in the incubator on the HoloMonitor stage. - Select Application
In the App Suite software, select the HoloMonitor Cell Proliferation Assay. - Select Wells
After selecting the assay, set up the experiment by selecting wells, imaging positions, duration and interval. - Start Experiment
Start image capturing. - View Results
During the experiment and while images are still being recorded, the results are shown on the computer screen in real-time. - Export Results
After the experiment is completed, export and use the final data to create other graphs or use the automatically created graphs in Excel. - Reuse Data
Optionally, reanalyze the recorded time-lapse images to quantify additional cellular characteristics by selecting a different HoloMonitor application.
How To Measure Cell Growth
The term “cell growth” can mean both growth of one cell during the cell cycle, growth of individual cells going through differentiation or it can mean cell culture growth which is also called cell proliferation. HoloMonitor is able to measure cell growth for the individual cell as well as for the cell population.
For all experiments, the cells are seeded in standard cell culture vessels. They are then placed on the HoloMonitor inside the incubator and the image capture is initiated. No labels or stains are necessary. The results are then analyzed using the Kinetic Cell Proliferation Assay or the In-depth Cell Morphology Assay.
References
- Croft et al., Digital Holographic Imaging as a Method for Quantitative, Live Cell Imaging of Drug Response to Novel Targeted Cancer Therapies, Theranostics (2019)
- Janicke et al., Label‐free high temporal resolution assessment of cell proliferation using digital holographic microscopy, Cytometry Part A (2017)
- Kamlund et al., Salinomycin Treatment Specifically Inhibits Cell Proliferation of Cancer Stem Cells Revealed by Longitudinal Single Cell Tracking in Combination with Fluorescence Microscopy, Applied Sciences (2020)