I enjoy spying on cells! As a Ph.D. candidate at Arnesen lab, this comes in handy when investigating the effect of N-terminal acetylation in eukaryotic cells.
Q: Tell us a bit more about your research!
A: I am a Ph.D. candidate at the Arnesen lab at the University of Bergen. The focus of our research group is N-terminal acetylation, a very common protein modification in eukaryotes. It is catalyzed by N-terminal acetyltransferases (NATs) which are linked to cancer and genetic syndromes.
I study the cellular effect of NatH, aka NAA80, which was recently revealed as an actin modifier. I use various imaging techniques to understand how this modification of actin plays a role in cancer cell migration and cell morphology. When it comes to live cell imaging, digital holographic imaging with HoloMonitor M4 is one of my main methods to study cancer cell behavior. In addition, I like to combine the quantitative population-based data from the HoloMonitor with high-resolution TIRF and 3D imaging of fixed and stained cells.
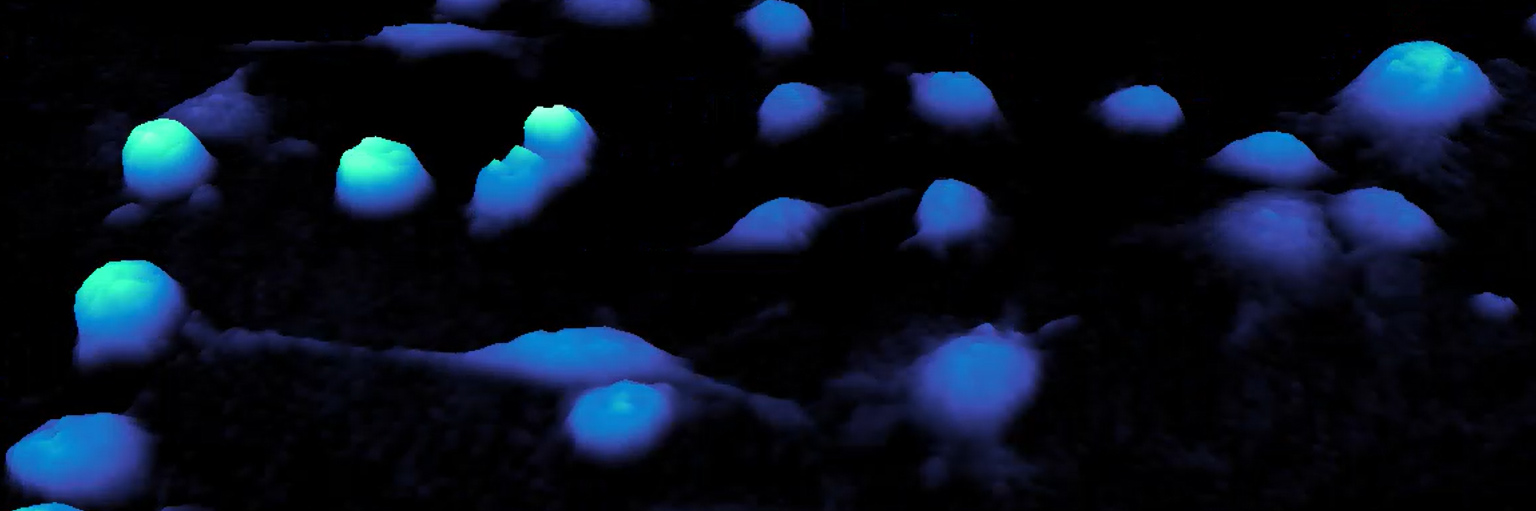
This HoloMonitor time-lapse shows a clump of suspension cells embedded (or trapped) in the Matrigel matrix. A single cell escapes the cell aggregate (or clump) and invades the 3D matrix.
Q: Can you describe how you typically use HoloMonitor in your work?
A: In our lab, the HoloMonitor M4 microscope is a “workhorse” when it comes to live cell imaging. It is ready to go in a designated incubator. The portfolio of integrated cell biological applications makes this a popular tool for many of our group members. I typically use HoloMonitor and its App Suite software on a regular basis to perform experiments and analyses on adherent cell cultures. Lately, we have been genetically modifying cancer cell lines. Here, I am using the HoloMonitor to assess the quality of my new clones.
Typically, to get an overview of some key parameters of the clonal populations, I will start a time-lapse at 10 min intervals, using the automatic Kinetic Cell Motility Assay in App Suite. Then, I just leave my cells with HoloMonitor for 2-3 days. (I always sneak peek at the real-time generated graphs!). When the time-lapse is done, I quickly re-analyze the images in the Kinetic Cell Proliferation Assay. Lastly, I pick a specific time point for the Cell Quality Control Assay. Re-analyzing only takes a few minutes with our computer set-up! I continue my clone evaluation by making some nice movies and still images and “violà”! Within a short time, the morphodynamics of my clones are revealed!
HoloMonitor time-lapse of a modified breast cancer cell line (MCF-7) with altered cytoskeleton dynamics.
Q: In your recent work1, you talk about 3D cell culture assays. How would you describe the importance of such assays and how is HoloMonitor fitting into this research?
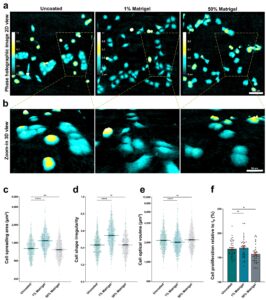
A: During my master’s project I was collecting data using the Wound healing Assay. My supervisor, Henriette Aksnes, had been brewing on an idea to combine this assay with a thin Matrigel overlay. By introducing this “third” dimension that mimic’s the extracellular space, the migrating cells would have to invade this matrix to close the gap.
When I discovered that the software was able to detect and “mask” the cells, without interference from the Matrigel, we were really excited! This was a breakthrough for us. It meant that we now had an efficient model to study metastatic properties. This methodical twist has been a very useful contribution to our toolbox of methods in order to study the invasive mode of populations and single cells.
For my Ph.D. colleague (“partner in crime”) and co-author, Hanne Øye, such Matrigel-based 3D assays have proven to be convenient for trapping & tracking suspension cells. It opens yet another usage of HoloMonitor and shows that assays with small spheroid-like cell clumps could indeed be performed with this instrument.
Q: Which features of the HoloMonitor system are important to you?
A: One important feature of the HoloMonitor system that I consider particularly important, is the gentle imaging technique. It secures the well-being of my cells. In addition, it allows for long-term monitoring of living cells combined with frequent automated imaging. In our lab, we often study new knockouts and in this work, the software’s General Capture function fits perfectly.
Here, we typically just run a video to see how the KOs differ from parental cells in terms of cellular parameters, without working from any hypothesis. For my project, I use a wide range of App Suite Assays for long-term studies of cellular behavior both in cell populations and at a single-cell level. Examples are the guided assays such as Kinetic Dose-Response, Kinetic Cell Motility, Kinetic Cell Proliferation. In addition, I use the in-depth analysis of Wound Healing, Single-cell Tracking for cell motility & migration, and Cell Morphology.
Wound healing assay of (modified) U2OS cells from Monica’s lab.
The Wound Healing Assay was the first one I tried, and I still use it heavily, especially after we figured out that it also works for wounds embedded in Matrigel in order to study cellular invasion. Since the HoloMonitor is quite busy in our lab, we have a designated “holo-running-computer” always connected to the microscope. In addition, in the same room, we have a neighbor “twin-computer,” which is used mainly for automatic and in-depth analysis that can be controlled remotely from home or the office/study hall.
On top of that, we have extra App Suite software licenses to avoid bottlenecks as we are a large and very active research group with many students. App Suite is also performing well from home on my gaming computer (AMD Ryzen Processor).
Q: Looking back at the first time you saw HoloMonitor live — what was your first impression?
A: Henriette enthusiastically introduced me to HoloMonitor M4 and the DHM live cell imaging method during my master’s project. At this time, the instrument was very new to us, and we were excited to be the pioneers in Norway. Previously, I had only been studying fixed cells using a conventional light microscope. Watching the color-paletted holographic 3D representation of the cells for the first time felt like pure magic! Indeed, by inspecting the videos, it immediately became very clear that my knockout had a much more motile phenotype. Also, it had a very dynamic morphology with larger lamellae causing bow-shaped cells that frequently shifted their polarity. You could say that they were dancing to another pace than the parental wild-type cells.
After a short training session with Henriette, I was able to run my own experiments and harvest quantitative cell biological data for my master’s thesis. Now, I am the instrument response and the one to train students. I find it intriguing how quickly new students without any experience with microscopy become comfortable handling the HoloMonitor.
Reference:
- Hellesvik et al. (2020) Exploiting the potential of commercial digital holographic microscopy by combining it with 3D matrix cell culture assays, Scientific Reports, 10:14680, https://doi.org/10.1038/s41598-020-71538-1.
By combining two powerful techniques, digital holographic microscopy using HoloMonitor M4 + 3D matrix embedded cells, we added a third dimension to our cancer cell research. This methodical advance enabled us trap & track cells in the matrix and thereby reveal morphological differences between migratory and invasive mode of cancer cells at the “leading edge”.
Monica Hellesvik,
University of Bergen
Read more from the Arnesen Lab in their blog:
Studying cells without a cell lab?
More flexibility in hands-on lab work and data analysis is something the world’s researchers have been looking for to adapt to the changing research environment in the eye of the covid-19 pandemic.
Indeed, you can re-analyze your data with App Suite. This way, you take your data analysis anywhere, anytime — swapping the lab bench with your living room.

The labs were still closed, and no additional experiments could be performed. Thus, the solutions had to come from our respective home offices. And here, the HoloMonitor’s built-in software solution showed its strength with a variety of applications and gave me the opportunity to re-analyze previous image material from home. New discoveries were made that strengthened our previous results.
Monica Hellesvik, Arnesen Lab, University of Bergen
Research from home blog post
We at PHI say Thank you to Monica and the Arnesen Lab for this interview!